Introduction:
Primary CNS lymphoma (PCNSL) is a highly aggressive non-Hodgkin lymphoma (NHL) variant limited to the CNS with rare evidence of systemic spread. It constitutes 4% of all CNS malignancies. Owing to the impenetrable blood-brain barrier to routine chemo-immunotherapy, the efficacy of such treatment is less than optimal. The combination of chemoradiotherapy has substantial associated risk of late neurotoxicity and increased disease recurrence. High dose chemotherapy (HDT) with autologous stem cell transplantation (ASCT) as rescue has been shown to be effective. We performed a systematic review of literature to explore the efficacy of HDT and ASCT in patients with PCNSL.
Methods:
We performed a comprehensive literature search (following PRISMA guidelines) on May 28, 2020 on Pubmed, Cochrane Library and Clinicaltrials.Gov by using the MeSH terms related to high-dose chemotherapy, autologous transplantation and primary CNS lymphoma which yielded 561 relevant articles. Following subsequent screening by 2 reviewers, we selected 16 published trials (n=745) and included data from these studies in our systematic review. We manually extracted data summarized the results.
Results:
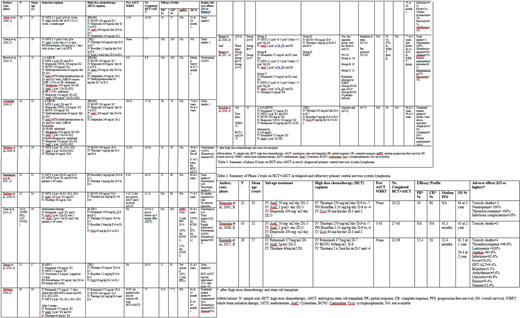
405/745 patients eventually underwent sequential HDT+ASCT the results of which are illustrated in table 1 and 2 stratified on the basis of newly diagnosed (ND) (n=641) and relapsed, refractory (RR) PCNSL patients (n=104) respectively.
ND PCNSL:
Among ND PCNSL patients, carmustine and thiotepa based regimens were the most widely studied HDT regimens (n= 351). Illerhaus et al. (2016, n= 81) in a phase 2 trial used HDT of intravenous (IV) combinations of rituximab, thiotepa and carmustine followed by ASCT on 73 patients which yielded partial response (PR) 13.9 %, complete response (CR) 77.2% and overall survival (OS) 81% at 36 months. 4 patients suffered transplant related mortality (TRM), mucositis (8%) and arrhythmias (2%) were the most common nonhematologic >grade 4 adverse effects. Ferreri et al. (2017) [n=122] in a phase 2 trial (n=59) used ASCT following Carmustine + Thiotepa HDT and reported 2-year OS of 71% and CR of 93% with a modest toxicity profile (infections (8%), mucositis (5%) and 2 (3%) treatment related deaths).
IV thiotepa, busulfan and cyclophosphamide (TBC) combination was used as HDT / ASCT in ND PCNSL patients (n=208). Omuro et al. (2015) (n=32) used HDT with TBC followed by ASCT in 26 patients. Whole brain radiation therapy (WBRT) was not given to any patient regardless of recurrence or relapse. At 5 years, the study reported PR=11%, CR=81%, and OS=81%. In a phase 2 trial, Houillier et al. (2019, n=140) used TBC combination as HDT followed by either WBRT or ASCT (2 separate arms), 44 patients of the latter arm exhibited a CR of 38% and OS of 66% at 5 years with 5 treatment related deaths.
An IV combinations of carmustine, etoposide, cytarabine and melphalan (BEAM) have also been used as HDT with ASCT in 3 phase 2 trials (n=59). Abrey et al. (2003, n=28) in a phase 2 trial used high-dose BEAM therapy followed by ASCT which yielded PR of 14%, CR of 57% and OS of 55% at 28 months. Only 1 treatment related mortality occurred.
RR PCNSL:
TBC combination has been the most extensively used HDT regimen for RR PCNSL patients undergoing ASCT (n=65). Soussain et al. (2008, n=43) conducted a phase 2 trial in which high-dose TBC sequentially followed by ASCT was studied in 27 RR PCNSL patients. The results showed median PFS of 41.1 months with OS of 45% at 2 years however, 3 toxicity related deaths were observed.
Kasenda et al. (2017, n=39) in a phase 2 trial used high-dose combination of rituximab, carmustine and thiotepa coupled with ASCT on RR PCNSL patients which yielded PR of 15.4%, CR of 56% and median PFS of 12.4 months with an OS of 56.4% at 2 years. However, there were 4 treatment related deaths and an extensive toxicity spectrum with widespread pancytopenia (thrombocytopenia =96.9%, leukopenia =100%, anemia =65.6%) and nonhematologic infections (65.6%) observed.
Conclusion:
HDT followed by ASCT rescue has exhibited favorable outcomes and can be used an alternative to WBRT especially in ND PCNSL patients. Evidence is limited by mainly phase II non randomized data. Although, hematologic adverse effects due to HDT were observed on a widespread basis, transplant related mortality was however minimal. There is need to carry out prospective randomized phase III trials to access the safety and efficacy profile of HDT / ASCT for ND and RR PCNSL.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal